URGENT!!!
The World Health Organization has declared MPox (formerly called Monkey Pox) as a Public Health Emergency, specifically in Africa.
Our Monkeypox Virus Antigen Rapid Test is a lateral flow immunoassay intended for the in vitro rapid, simultaneous qualitative detection of human monkeypox virus antigens in oropharyngeal swab, and exudate of blain swab specimen to aid in the diagnosis of Monkeypox Virus infection.
Specification
★ High Sensitivity and Specificity, suitable for early stage detection
★ Fast Reaction: 15 minutes
★ CE mark
Performance
Sensitivity (Positive Percent Agreement): 99.08% = 108/109 (95% CI: 94.99%~99.84%)
Specificity (Negative Percent Agreement): 99.67% = 309/310 (95% CI: 98.20%~99.94%)
Accuracy (Overall Percent Agreement): 99.52% = (108+309)/419 (95% CI: 98.28%~99.87%)
Meet Sure Bio-Tech at Medlab 2024
Welcome to visit us at stand Z3.C11 on 5-8, Feb., 2024 in Dubai.

MEDICA IMPRESSION
It is an annual opportunity to meet new and old friends at MEDICA! Looking forward to the next reunion!
We are ready whenever you need a reliable IVD products supplier!

New Products Launching – Fluorescence Immunoassay
Immunofluorescence Analyzer is an advanced fluorescence immunoassay analyzing instrument intended for use to provide an accurate diagnosis aid in conditions such as cardiovascular diseases, thyroid function, diabetes, sex hormones, tumors, infections etc.
Features
- Portable Design
- Easy to Use
- Wide Test Items
- LIS/HIS connectivity
Wide Test Items
| Cardiac Marker | cTnI, Myoglobin, CK-MB, NT-proBNP, D-Dimer, cTnI /Myoglobin/CK-MB |
| Inflammation Marker | PCT, IL-6, CRP, SAA |
| Diabetes | HbA1c |
| Health Check | 25-OH VD, Ferritin, VB12, Folic acid |
| Tumor Marker | CEA, fPSA, PSA, FOB |
| Hormones Marker | AMH, LH, FSH, PRL, Testosterone, Progesterone, Estradiol, βHCG |
| Thyroid Marker | TSH, T3, T4, FT3, FT4 |
| Infectious Disease | HBsAg, HCV, HIV, TP, Flu A&B, Dengue Ag/Ab, SARS-CoV-2 Ag |
Meet Sure Bio-Tech at MEDICA 2023
Welcome to visit us at stand No. J62-1 in Hall No. 16.

About the Event
Delve into the world of medical technology! The whole industry will meet at MEDICA in Düsseldorf 13-16 November 2023. Experience high-tech products, meet world market leaders, hidden champions and start-ups or visit the top-class forums and conferences. Regardless what you are looking for: you will find it at MEDICA 2023!
Sure Bio-Tech at MEDEXPO AFRICA
Reunion with old friends and made new friends.
Meet us at MEDEXPO AFRICA in KENYA
Welcome to visit our booth No. 104 at the MEDEXPO AFRICA in Nairobi from the 21st to the 23rd of June 2023.

ABOUT THE EVENT
Strong exhibitor line-up from over 25 countries and a comprehensive medical product, equipment & machinery showcase at Kenya’s biggest International Medical Trade Exhibition.
The increasing demand for medical manufacturing products, equipment, machinery, services and solutions has prompted a surge of representation at Africa’s premier medical exhibition, MEDEXPO AFRICA – KENYA 2023. Taking place at Sarit Expo Center, Nairobi , Kenya, from 21 – 23 June, 2023, the exhibition profiles innovative solutions from leading market players for the benefit of buyers from the medical technology industry, from across the East African region. Targeted buyers from across East Africa are seeking new products, equipment, machinery, services and solutions to keep abreast of industry trends and developments.
Newly Launching:
Epstein Barr Virus (EBV) is directly associated with various human cancers of epithelial, mesenchymal and especially lymphoid origin inside whose cells can be identified. The International Agency for Research on Cancer (IARC) lists EBV as a human carcinogen.
VC® EBER Detection Kit (CISH) is intended for the detection of nucleic acid targets on in-situ hybrid cell tissue, to provide physicians with auxiliary information for diagnosis.
VC® EBER/CD3 Dual-staining Detection Kit is intended for the In-situ hybridization and immunohistochemical staining on the basis of HE staining, to provide physicians with auxiliary information for diagnosis.
Newly Launching:
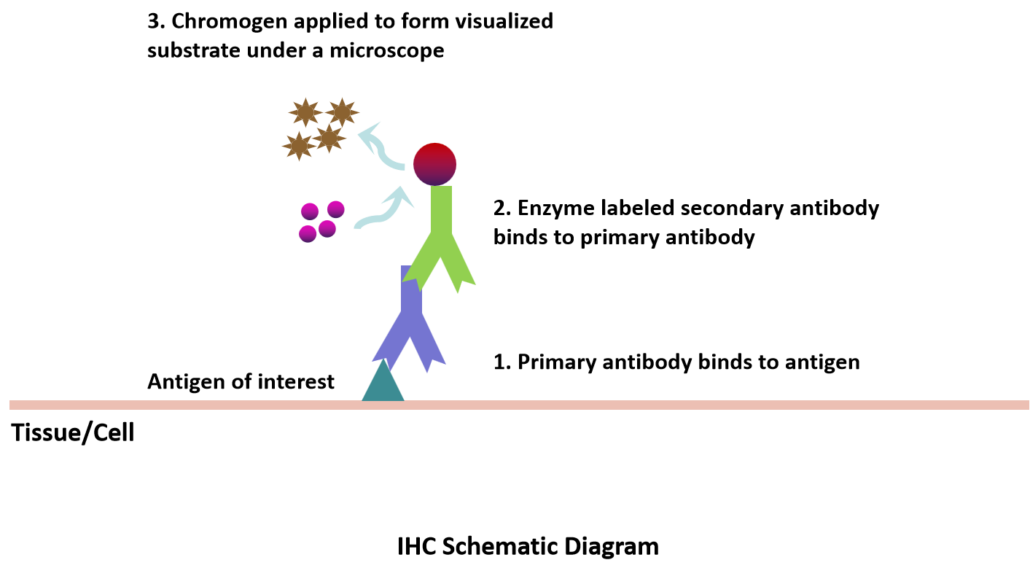
VC® Primary Antibody provides large portfolio of high-quality primary antibodies that bind target proteins or other relevant antigens specifically and sensitively.
VC® Secondary Antibody provides poly-HRP and poly-AP labelled secondary antibodies intended for chromogenic detection of targeted antigens that have been reacted to a user-supplied primary antibody on human tissue.
VC® p16/Ki-67 Dual-staining Detection Kit is a qualitative immunocytochemical assay intended for the simultaneous detection of the p16 and Ki-67 proteins in human tissue specimen.
Newly Launching:
The Auto Stainer uses the filtering principle, which is suitable for slide making and staining for the specimens of sputum, pleural effusion, urine, cerebrospinal fluid, cervical cancer cells and etc. The Auto Stainer provides semi-automated (MS-1, MS-5) and automated (MS-AUTO20) staining function and must be operated by professionals or trained laboratorians.
The Interlayer Acid-Fast Bacilli Test Kit incorporated with Concentrated Sandwich Smear Method, is intended for use in the screening of acid-fast bacilli present in various body fluid specimens. By working with Auto Stainer, the sample can be concentrated and stained on a membrane to improve detecting rate.
With AFB Testing System, let’s get closer to STOP TB!
Newly Launching:
| Rapid Test | |
| ☼ | Chagas |
| ☼ | Chikungunya |
| ☼ | Strep A |
| ☼ | Mononucleosis |
| ☼ | ZIKA virus |
| ☼ | Clostridium Difficile |
| ☼ | Legionella Pneumophila |
| ☼ | Leptospira |
| ☼ | Entamoeba histolytica |
| ☼ | Giardia Iamblia |
| ☼ | Cryptosporidium |
| ☼ | Filariasis |
| ☼ | EV 71 IgM |
| ☼ | V.cholerae O1/O139 |
| ☼ | Ebola |
| ☼ | Respiratory Combo (COVID-19/RSV/ADV/FLU) |
Newly Launching:
Monkeypox Virus Antigen Rapid Test
Intended Use
The test is intended for in vitro qualitative detection of monkeypox virus(MPXV) antigen in human oropharyngeal swab, whole blood or skin lesion materials, including lesion exudate, lesion roofs, lesion crusts.
Specification
★ High Sensitivity and Specificity, suitable for early stage detection
★ Fast Reaction: 15 minutes
★ CE mark
Performance
The minimum detection limit of the test for detecting the monkeypox
recombinant antigen is 100pg/ml.
Ordering Information
| Products | Specimen | Format | Cat. No. | Tests/ kit | Note |
| Monkeypox Virus Antigen Rapid Test | Swab/Lesion roofs, Lesion crusts/whole blood | Card * | VC019201-CE-1 | 25 | CE marked |
Newly Launching:
Monkeypox Virus IgG/IgM Antibody Rapid Test
Intended Use
The test is intended for in vitro qualitative detection of monkeypox IgM and IgG antibodies in human serum, plasma, whole blood or fingerstick whole blood sample qualitatively
Specification
★ High Sensitivity and Specificity, suitable for early stage detection
★ Fast Reaction
★ CE Marked
Ordering Information
| Products | Specimen | Format | Cat. No. | Tests/ kit |
| Monkeypox Virus Antigen Rapid Test | Whole blood | Card | VC019201-CE-2 | 25 |
| * CE marked |
Newly Launching:
2019‐nCoV antigen and Flu A/B combo Rapid Test
(Colloidal Gold)
The 2019- nCoV Antigen rapid test is used for in vitro qualitative detection of the antigen of novel coronavirus in human throat swabs or nasal swabs.
The Flu A/B rapid Test is a colloidal gold enhanced, rapid immunoassay for the qualitative detection of influenza A and influenza B viral nucleoprotein antigens. The test is intended for healthcare professional use onl
Specification
★ High Sensitivity and Specificity, suitable for early stage detection
★ Fast Reaction
★ CE certified
Performance
For 2019- nCoV Antigen rapid test
| 2019-nCoV Antigen Rapid Test | PCR Test | Total | ||
| Positive | Negative | |||
| Positive | 74 | 2 | 76 | |
| Negative | 6 | 238 | 244 | |
| Total | 80 | 240 | 320 | |
Analysis of coincidence rate of 2019- nCoV Antigen rapid test and PCR Test in nasal samples:
Positive coincidence rate (Sensitivity): 74/ (74+6) × 100% = 92.5%
Negative coincidence rate (Specificity): 238 / (2+238) × 100% = 99.16%
Total coincidence rate (Accuracy):
(74+238) / (74+6+2+238) × 100% = 97.5%
For 2019- nCoV Antigen rapid test
Flu A Sensitivity and Specificity
| Flu A Rapid Test | PCR Test | Total | ||
| Positive | Negative | |||
| Positive | 163 | 16 | 179 | |
| Negative | 12 | 990 | 1002 | |
| Total | 175 | 1006 | 1181 | |
Positive coincidence rate (Sensitivity): 163/ (163+12) × 100% = 93.14%
Negative coincidence rate (Specificity): 990/ (16+990) × 100% = 98.41%
Total coincidence rate (Accuracy):
(163+990) / (163+12+16+990) × 100% = 97.63%
Flu B Sensitivity and Specificity
| Flu B Rapid Test | PCR Test | Total | ||
| Positive | Negative | |||
| Positive | 195 | 15 | 210 | |
| Negative | 12 | 1050 | 1062 | |
| Total | 207 | 1065 | 1272 | |
Positive coincidence rate (Sensitivity): 195/ (195+12) × 100% = 94.20%
Negative coincidence rate (Specificity): 1050/ (15+1050) × 100% = 98.59%
Total coincidence rate (Accuracy):
(195+1050) / (195+12+15+1050) × 100% = 97.87%
Newly Update:
2019-nCov Antigen Rapid Test
(Colloidal Gold)
New version product is used for in vitro qualitative detection of the antigen of novel coronavirus in human throat swabs or nasal swabs or saliva
Specification
★ High Sensitivity and Specificity, suitable for early stage detection
★ Fast Reaction
★ CE certified
Performance
For swabs specimen
| 2019-nCoV Antigen Rapid Test | PCR Test | Total | ||
| Positive | Negative | |||
| Positive | 74 | 2 | 76 | |
| Negative | 6 | 238 | 244 | |
| Total | 80 | 240 | 320 | |
Analysis of coincidence rate of 2019-nCoV Antigen rapid test and PCR Test in nasal samples:
Positive coincidence rate (Sensitivity):
74/ (74+6) × 100% = 92.5%
Negative coincidence rate (Specificity):
238 / (2+238) × 100% = 99.16%
Total coincidence rate (Accuracy):
(74+238) / (74+6+2+238) × 100% = 97.5%
For saliva specimen
| 2019-nCoV Antigen Rapid Test | PCR Test | Total | ||
| Positive | Negative | |||
| Positive | 65 | 2 | 67 | |
| Negative | 7 | 250 | 257 | |
| Total | 72 | 252 | 324 | |
Analysis of coincidence rate of 2019-nCoV Antigen rapid test and PCR Test in saliva samples:
Positive coincidence rate (Sensitivity):
65/ (65+7) × 100% = 90.28%
Negative coincidence rate (Specificity):
250/ (2+250) × 100% = 99.20%
Total coincidence rate (Accuracy):
(65+250) / (65+7+2+250) × 100% = 97.22%
Newly Launching:
2019-nCov Antigen Rapid Test
(Colloidal Gold)
This product is used for in vitro qualitative detection of the antigen of novel coronavirus in human throat swabs or nasal swabs.
Specification
★ High Sensitivity and Specificity, suitable for early stage detection
★ Fast Reaction
★ CE certified
Performance
| 2019-nCoV Antigen Rapid Test | PCR Test | Total | ||
| Positive | Negative | |||
| Positive | 74 | 2 | 76 | |
| Negative | 6 | 238 | 244 | |
| Total | 80 | 240 | 320 | |
Analysis of coincidence rate of 2019- nCoV Antigen rapid test and PCR Test in nasal samples:
Positive coincidence rate (Sensitivity):
74/ (74+6) × 100% = 92.5%
Negative coincidence rate (Specificity):
238 / (2+238) × 100% = 99.16%
Total coincidence rate (Accuracy):
(74+238) / (74+6+2+238) × 100% = 97.7%
Newly Launching:
Diagnostic kit for SARS-COV-2 Ag Test
(Fluorescence Immunochromatographic Assay)
The kit is used for in vitro qualitative detection of SARS-CoV-2 antigenin human nasopharyngeal swabs and oropharyngeal swabs samples.
Specification
★ High Sensitivity and Specificity, suitable for early stage detection
★ Fast Reaction
★ CE certified
Performance
- Site 1 (Oropharyngeal swab eluted with matched sample extraction solution)
| CT values | Number of samples | 2019 nCoV RT-PCR Results | Sure Biotech SARS-CoV-2 antigen test result as compared to RT-PCR |
| ≤30 | 57 | pos | 57/57=100% |
| ≤33 | 82 | pos | 82/82=100% |
| ≤34 | 91 | pos | 90/91=98.90% |
| ≤36 | 149 | pos | 136/149=91.28% |
| ≥40 | 45 | neg | 44/45=97.78% |
- Site 2 (Nasopharyngeal swab/Oropharyngeal swab kept in UTM)
| CT values | Number of samples | 2019 nCoV RT-PCR Results | Sure Biotech SARS-CoV-2 antigen test result as compared to RT-PCR |
| ≤25.1 | 52 | pos | 52/52=100% |
| ≤34.8 | 82 | pos | 77/82=93.9% |
| >40 | 45 | neg | 45/45=100% |
Due to sampling deviations, differences in the infection progress for patients, and operational deviations, these data are only responsible for the results of respective clinical trials.
A negative test result may occur if the level of antigen in a sample is below the detection limit of the test or if the sample was collected or transported improperly.
Newly Launching:
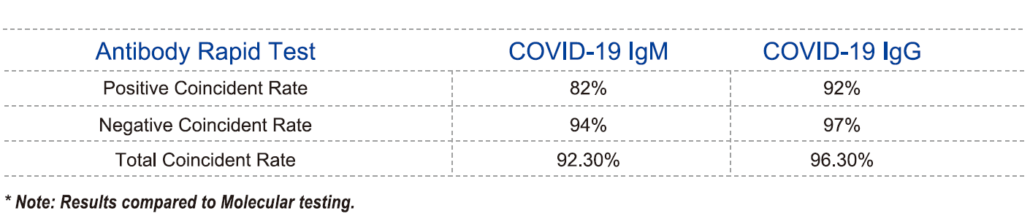
Novel Coronavirus (COVID-19) IgM/IgG Ab Rapid Test
Used for the qualitative detection of the antibody IgM/IgG to novel Coronavirus (COVID-19) in human serum/
plasma/whole blood.
Specification
★ Sample Volume: 10 μL
★ Fast Reaction: 15 minutes
★ High Coincidence Rate with PCR test
Performance
Exhibiting atMedlab in Feb, 2020 in UAE, DUBAI, meet with our partners/friends
Exhibiting at Arab Health and participating at Medlab in Jan/Feb, 2019 in UAE, DUBAI, meet with our partners/friends
2018
World AIDS Day, 1st Dec. 2018
 Fact sheets of AIDS:
Fact sheets of AIDS:
- HIV continues to be a major global public health issue, having claimed more than 35 million lives so far. In 2017, 940 000 people died from HIV-related causes globally.
- There were approximately 36.9 million people living with HIV at the end of 2017 with 1.8 million people becoming newly infected in 2017 globally.
- 59% of adults and 52% of children living with HIV were receiving lifelong antiretroviral therapy (ART) in 2017.
- The WHO African Region is the most affected region, with 25.7 million people living with HIV in 2017. The African region also accounts for over two thirds of the global total of new HIV infections.
- HIV infection is often diagnosed through rapid diagnostic tests (RDTs), which detect the presence or absence of HIV antibodies. Most often these tests provide same-day test results, which are essential for same day diagnosis and early treatment and care.
- There is no cure for HIV infection. However, effective antiretroviral (ARV) drugs can control the virus and help prevent transmission so that people with HIV, and those at substantial risk, can enjoy healthy, long and productive lives.
- Between 2000 and 2017, new HIV infections fell by 36%, and HIV-related deaths fell by 38% with 11.4 million lives saved due to ART in the same period. This achievement was the result of great efforts by national HIV programmes supported by civil society and a range of development partners.
Fact sheet Source: www.who.int
What Sure biotech could offer for YOU with high quality HIV dignostic products:
Rapid test:
- HIV 1/2 Rapid Test —detection of HIV antibodies
- HIV 1/2 Tri-line Rapid Test—to test HIV 1 and HIV 2 seperately
- HIV POCT Rapid Test — individual packing, with safety lancets, swabs for single using in home or other sites
- HIV ag/ab Rapid Test — 4th generation, detection of the antibodies to all isotypes(IgG, IgM and IgA) specific to HIV-1(including subtype-O), and HIV-2 and p24 antigen
- HIV 1/2 Oral Rapid Test —Oral detection of HIV antibodies
- HIV 1/2 Rapid Test (CE version) — CE certifified HIV version
For more details, pls see: https://www.surebiotech.com/rapid-test/rapid-infectious-disease-test/#toggle-id-3
Elisa test:
- HIV Elisa test —detection of HIV antibodies
- HIV ag/ab Elisa test— 4th generation, detection of the antibodies and p24 antigen
For more details, pls see: https://www.surebiotech.com/elisa-test/elisa-infectious-disease-test/#toggle-id-3
2018
HIV Ag/Ab Combo Rapid Test
 New Products Launched:
New Products Launched:
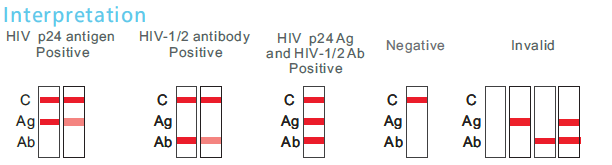
VC® HIV Ag/Ab Combo Rapid Test (HIV 4th generation) is a colloidal gold enhanced, rapid immunochromatographic assay for the qualitative detection of the antibodies to all isotypes(IgG, IgM and IgA) specific to HIV-1(including subtype-O), and HIV-2 and p24 antigen in human serum, plasma or whole blood. Within 20 minutes, the result appear on the membrane. It is one step,rapid, easy-to-use test.
| Performance | ||
| Detect | Sensitivity | Specificity |
| HIV Antibody | 100% | 99.50% |
| HIV p24 Antigen | 100% | 99.20% |
| Ordering information | |||||
| Cat. No. | Description | Type | Specimen | Pack size | Note |
| VC010304 | HIV Ag/Ab Combo Rapid Test | Cassette | WB/S/P | 25T/Kit | |
| VC010305 | HIV Ag/Ab Combo Rapid Test | Strip | WB/S/P | 50T/Kit | |
| VC010306 | HIV Ag/Ab Combo Rapid Test | Cassette | WB/S/P | 25T/kit | with 25 lancets and swabs |
| VC010307 | HIV Ag/Ab Combo Rapid Test | Cassette | WB/S/P | 1T/kit | with safety lancet and swab, single packing |
2018
World Hepatitis Day, 28 July
![]() Fact sheets of Hepatitis:
Fact sheets of Hepatitis:
HAV:
- Epidemics can be explosive and cause substantial economic loss
- A safe and effective vaccine is available to prevent hepatitis A.
HBV:
- An estimated 257 million people are living with hepatitis B virus infection (defined as hepatitis B surface antigen positive).
- In 2015, hepatitis B resulted in 887 000 deaths, mostly from complications (including cirrhosis and hepatocellular carcinoma).
- it can be prevented by currently available safe and effective vaccine.
HCV:
- Globally, an estimated 71 million people have chronic hepatitis C infection. A significant number of those who are chronically infected will develop cirrhosis or liver cancer.
- Approximately 399 000 people die each year from hepatitis C, mostly from cirrhosis and hepatocellular carcinoma.
- There is currently no vaccine for hepatitis C; however research in this area is ongoing.
HDV:
- At least 5% of people with chronic HBV infection are co-infected with HDV, resulting in a total of 15 – 20 million persons infected with HDV worldwide. However, this is a broad global estimation since many countries do not report the prevalence of HDV
- Hepatitis D infection can be prevented by hepatitis B immunization
HEV:
- Every year, there are an estimated 20 million HEV infections worldwide, leading to an estimated 3.3 million symptomatic cases.
- WHO estimates that hepatitis E caused approximately 44 000 deaths in 2015 (accounting for 3.3% of the mortality due to viral hepatitis).
- A vaccine to prevent hepatitis E virus infection has been developed and is licensed in China
Fact sheet Source: www.who.int
What Sure biotech could offer for YOU with high quality products:
Rapid test: rapid HAV test, rapid HBV test, rapid HCV test, rapid HEV test
Elisa test: Elisa HAV test, Elisa HBV test, Elisa HCV test, Elisa HEV test
Vaccine: HAV Vaccine, HBV Vaccine, HEV Vaccine (from our partner of GMP manufacturers)
2018
East Africa visit 2018 in June in Ethiopia, Kenya, Tanzania, Uganda, meet with our partners and friends
2018
Middle East visit 2018 in May in Egypt, Lebanon, Jordan, meet with our partners and friends
2018
CMEF 2018 in April in Shanghai, China, meet with our partners and friends
2018
CACLP 2018 in March in Chongqing, China, meet with our partners and friends
2017
CMEF 2017 in May in Shanghai, China, meet with our partners and friends

2017
World Malaria Day, 25 April

Case:
According to the WHO report, there were 212 million new cases of malaria worldwide in 2015 (range 148–304 million). The WHO African Region accounted for most global cases of malaria (90%), followed by the South-East Asia Region (7%) and the Eastern Mediterranean Region (2%).
In 2015, there were an estimated 429 000 malaria deaths (range 235 000–639 000) worldwide. Most of these deaths occurred in the African Region (92%), followed by the South-East Asia Region (6%) and the Eastern Mediterranean Region (2%).
Children under 5 are particularly susceptible to malaria illness, infection and death. In 2015, malaria killed an estimated 303 000 under-fives globally, including 292 000 in the African Region. Between 2010 and 2015, the malaria mortality rate among children under 5 fell by an estimated 35%. Nevertheless, malaria remains a major killer of under-fives, claiming the life of 1 child every 2 minutes.
Diagnostics
WHO recommends diagnostic testing for all people with suspected malaria before treatment is administered. Rapid diagnostic testing (RDTs), introduced widely over the past decade, has made it easier to swiftly distinguish between malarial and non-malarial fevers, enabling timely and appropriate treatment.
New data presented in the report show that, in 2015, approximately half (51%) of children with a fever who sought care at a public health facility in 22 African countries received a malaria diagnostic test compared to 29% in 2010. Sales of RDTs reported by manufacturers rose from 88 million globally in 2010 to 320 million in 2013, but fell to 270 million in 2015.
Funding trends
In 2015, malaria funding totalled US$ 2.9 billion, representing only 45% of the GTS funding milestone for 2020. Governments of malaria-endemic countries provided 32% of total funding. The United States of America and the United Kingdom are the largest international funders of malaria control and elimination programmes, contributing 35% and 16% of total funding, respectively. If the 2020 targets of the GTS are to be achieved, total funding must increase substantially.
Source: http://www.who.int/malaria/media/world-malaria-report-2016/en/
2017
Arab Health/Arab Lab 2017 in Dubai, meet with our partners there.

2017
Renewed website
2016
Medical show in Germany, business meeting with our customers

2016
Business Visit for Kenya, Tanzania, Nigeria, Uganda
2016
Business Visit for Thailand, Indonesia

2016
Company team-building program

2016
Business Visit for Belgium, Portugal
2015
Medical show in Germany, business meeting with our customers

2015
AACC show in USA, business meeting with our customers

2015
Business Visit for SA, Mozambique, Zimbabwe
2015